Leukemia cutis (LC) is a rare cutaneous infiltration of extramedullary neoplastic hematopoietic cells with a paucity of data on their management, given that most cases are from individual case reports or case series, which further impact outcomes. This review aims to investigate the clinical characteristics of LC and highlight our cytogenetic findings that could contribute to our growing understanding and help reshape the prognosis of this rare but deadly condition.
Patients and methods
PubMed, Medline, ScienceDirect, and Scopus databases searched for "Leukemia Cutis" case reports from January 2000 to July 2020 pooled with a case from our institution.
Results
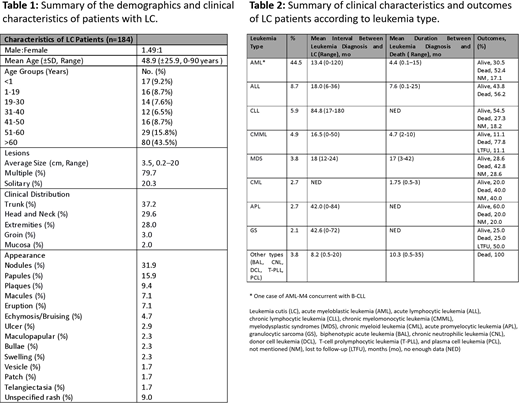
We included 184 biopsy-proven LC cases. Male: female ratio was 1.49:1. Mean age at diagnosis (± SD) was 48.9 (±25.9) years. Regarding age groups, 17 (9.2%), 16 (8.7%), 14 (7.6%), 12 (6.5%), 16 (8.7%), 29 (15.8%), and 80 (43.5%) patients were noted in the age groups of <1, 1-19, 19-30, 31-40, 41-50, 51-60, and >60 years, respectively. The demographics and clinical features of LC are summarized in Table 1.
The presenting finding was LC in 66 (35.8%) cases, with an average time-to-leukemia diagnosis of 8.1 months (range, five days-72 months), and aleukemic LC (ALC) in 17 (9.2%) cases. Mean leukemia-to-LC diagnosis interval was 25.6 (range, 0-180 months). Acute myeloblastic leukemia (AML) was the most common type, found in 82 (44.5%) cases, out of which, M5, M4, and M2 variants were predominant at 21.9%, 9.7%, and 9.7%, respectively. Sixteen (8.7%) cases were secondary to acute lymphocytic leukemia (ALL), out of which 56.2% were B-cell lineage. Eleven (5.9%) cases were secondary to chronic lymphocytic leukemia (CLL). Other less common types were chronic myelomonocytic leukemia (CMML), myelodysplastic syndromes (MDS), chronic myeloid leukemia (CML), and acute promyelocytic leukemia (APL) in 9 (4.9%), 7 (3.8%), 5 (2.7%), and 5 (2.7%), respectively.
The most common treatment modality was chemotherapy in 109/133 (81.9%) cases with the available data, out of which, 80 (73.4%) had chemotherapy alone, 16 (14.7%) had chemotherapy plus stem cell transplantation (SCT), 8 (7.3%) had chemotherapy plus radiotherapy, 3 (2.8%) had chemotherapy plus surgery and radiotherapy, 1 (0.9%) had chemotherapy plus radiation and SCT, and 1 (0.9%) had chemotherapy plus surgery. Mean duration of follow-up was 11 months (range, 1 day-100 months). In terms of outcomes, 61 (33.15%) patients were alive upon follow-up, out of which, 19 (31.1%) in remission, 18 (29.5%) disease-free, 17 (27.9%) with persistent disease, 2 (3.3%) had a recurrence, and 5 (8.2%) outcome not mentioned. Moreover, 91 (49.46%) patients died from disease. For the reported data, the average interval from leukemia diagnosis to death was 4.4 months (range, 3 days-15 months) for AML and 7.6 months (range, four days-25 months) for ALL (Table 2). Interestingly, at the time of our patient's LC diagnosis in our institution, cytogenic analysis revealed a novel inv(11)(p15q23) chromosomal aberration that herald MDS-to-AML transformation.
Discussion
In this review, several findings are noteworthy. First, males were more commonly affected. Second, 109 (59.2%) patients were older than 50 years of age. Third, LC noted as the initial presentation of systemic disease in more than a third of patients, whereas ALC was the initial presentation in only around 9% of patients. Fourth, multiple cutaneous lesions were more prevalent, with nodules being the most common presentation. Fifth, AML was the most predominant type overall, found in almost 45% of cases, followed by ALL, CLL, CMML, and other less common types. Sixth, chemotherapy was the most common treatment modality overall in more than 80% of patients with reported data. Seventh, almost half of patients died from the disease or complications related to it. Distinctly, very few studies in the literature reported this unique AML association with chromosome 11 aberrations.
Conclusion
LC is relatively rare and has a dismal prognosis. It most likely presents as the initial manifestation of leukemia, and physicians could easily misdiagnose this condition if managed without a biopsy. In this study, we intend to promote early recognition among physicians and highlight our unique cytogenetic findings. This could support future endeavors and develop novel patient-specific therapeutic strategies that exploit chromosomal aberrations amidst possible leukemogenic mechanisms.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.